Abstract
Research Article
Novel European Asiatic Clinical, Laboratory, Molecular and Pathobiological (2015-2020 CLMP) criteria for JAK2V617F trilinear polycythemia vera (PV), JAK2exon12 PV and JAK2V617F, CALR and MPL515 thrombocythemias: From Dameshek to Constantinescu-Vainchenker, Kralovics and Michiels
Jan Jacques Michiels*, King H Lam, Fibo Ten Kate, Dong-Wook Kim, Myungshin Kim, Vasily Shuvaev, Francisca Valster, Vincent Potters, Wilfried Schroyens, Mihaela Andreescu, Adrian Trifa, Achille Pich and Hendrik De Raeve
Published: 03 April, 2020 | Volume 3 - Issue 1 | Pages: 001-020
The Myeloproliferative Neoplasms (MPN) of trilinear polycythemia vera (PV) and megakaryocytic leukemia (ML = primary megakaryocytic granulocytic myeloproliferation: PMGM) and Essential Thrombocythemia (ET) in the studies of Dameshek and Michiels are caused by the MPN driver mutations JAK2V617F, JAK2exon12, CALR and MPL515 discovered by Constantinescu-Vainchenker, Green and Kralovics. The JAK2V617F mutated trilinear myeloproliferative neoplasms (MPN) include a broad spectrum of clinical laboratory and bone marrow features in essential thrombocythemia (ET), prodromal PV and erythrocythemic PV, classical PV and advanced stages of masked PV and PV complicated by splenomegaly and secondary myelofibrosis (MF). Heterozygous JAK2V617F mutated ET is associated with low JAK2 allele and MPN disease burden and normal life expectance. In combined heterozygous and homozygous or homozygous JAK2V617F mutated trilinear PV, the JAK2 mutation load increases from less than 50% in prodromal PV and classical PV to above 50% up to 100% in hypercellular PV, advanced PV and PV with MF. Bone marrow histology show diagnostic features of eryhrocytic, megakaryocytic and granulocytic (EMG) myeloproliferation in JAK2V617F mutated trilinear MPN, which clearly differs from monolinear megakaryocytic (M) myelproliferation in MPL and CALR thrombocythemia and dual megakaryocytic granulocytic (MG) myeloproliferation in CALR mutated thrombocythemia. The morphology of clustered large pleomorphic megakaryocytes with hyperlobulated nuclei are similar in JAK2V617F thrombocythemia, prodromal PV and classical PV patients. Monolinear megakaryocytic (M) myeloproliferation of large to giant megakaryocytes with hyperlobulated staghorn-like nuclei is the hallmark of MPL515 mutated normocellular thrombocythemia. CALR mutated thrombocythemia usually presents with high platelet count around 1000x109/l and normocellular megakaryocytic (M) proliferation of immature megakaryocytes with cloud-like hyperchromatic nuclei followed by dual megakaryocytic granulocytic (MG) myeloproliferation followed by various degrees of bone marrow fibrosis. Natural history and life expectancy of MPN patients are related to the response to treatment and the degree of anemia, splenomegaly, myelofibrosis and constitutional symptoms. The acquisition of epigenetic mutations at increasing age on top of MPN disease burden independently predict unfavorable outcome in JAK2V617F, MPL515 and CALR mutated myeloproliferative neoplasms (MPNs, which mutually exclude each other).
Read Full Article HTML DOI: 10.29328/journal.ijbmr.1001011 Cite this Article Read Full Article PDF
Keywords:
Myeloproliferative neoplasms; Essential thrombocythemia; Polycythemia vera; Primary megakaryocytic granulocytic myeloproliferation; Myelofibrosis; JAK2V617F mutation; MPL515 mutation; Calreticulin mutation; JAK2 wild type; Bone marrow histology
References
- Dameshek W, Henstell HH. The diagnosis of polycythemia. Ann Intern Med. 1940; 13: 1360-1387.
- Dameshek W. Physiopathology and course of polycythemia vera as related to therapy. JAMA. 1950; 142: 790-797. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15405984
- Videbak A. Polycythemia vera. Course and prognosis. Acta Med Scand. CXXXVIII. 179-197.
- Dameshek W. The treatment of Polycythemia. Blood. 1946; 1: 256.
- Michiels JJ. Physiopathology, etiologic factors, diagnosis and course of polycythemia vera as related to therapy according to William Dameshek 1940-1950. Turk J Hematol. 2013; 30: 102-110. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24385771
- Michiels JJ, De Rave H, Valster F, Potters V, Kim Y, et al. Extension of 2016 World Health Organization (WHO) classification and a new set of clinical, laboratory, molecular and pathological criteria for the diagnosis of myeloproliferative neoplasms: from Dameshek to Vainchenker, Green and Kralovics. EMJ. 2017a; 2: 72-81.
- Michiels JJ, Berneman Z, Gadisseur A, Raeve HD, Schroyens W, et al. Myelofibrosis is a Secondary Event in JAK2 Trilinear Myeloproliferative Neoplasm (MPN) and in CALR and MPL Thrombocythemia: Implications for Novel Treatment Options of Prefibrotic MPN. J Hematol Thromembolic Dis. 2017b; 5: 5.
- Michiels JJ, Hendrik De Raeve, Berneman Z, Van Bockstaele D, Hebeda K, et al. The 2001 world health organization and updated european clinical and pathological criteria for the diagnosis classification and staging of the Philadelphia-negative chronic myeloproliferative disorders. Sem Thromb Hemost. 2006a; 32: 307-340.
- Michiels JJ, Berneman Z, Van Bockstaele D, Van Der Planken M, De Raeve H, et al. Clinical and laboratory features, pathobiology of platelet-mediated thrombosis and bleeding complications and the molecular etiology of essential thrombocythemia and polycythemia vera: therapeutic implications. Sem Thromb Hemost. 2006b; 32: 174-207. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16673274
- Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951; 6: 372-375. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14820991
- Georgii A, Vykoupil KF, Buhr T, Choritz H, Döhler U, et al. Chronic myeloproliferative disorders in bone marrow biopsies. Path Res Pract. 1990; 186: 3-27.
- Georgii A, Buhr T, Buesche G, Kreft A, Choritz H. Classification and staging of Ph-negative myeloproliferative disorders by histopathology from bone marrow biopsies. Leuk Lymphoma. 1996; 22: 15-29. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8951769
- Michiels JJ, Prins MEF, Hagemeijer A, Brederoo, P, van der Meulen J, et al. Philedelphia chromosome positive essential thrombocythemia and megakaryoblast leukemia. Am J Clin Pathol. 1987; 88: 645-752. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3479003
- Michiels JJ. Diagnostic criteria of the myeloproliferative disorders (MPD): essential thrombocythemia (ET), polycythemia vera (PV) and chronic megakaryocytic granulocytic metaplasia (CMGM). Neth J Med. 1997; 51: 57-64. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9286142
- Michiels JJ, Thiele J. Clinical and pathological criteria for the diagnosis of essential thrombocythemia, polycythemiavera and idiopathic myelofibrosis (agnogenic myeloid metaplasia). Int J Hematol. 2002; 76: 133-145. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12215011
- Michiels JJ, Kutti J, Stark P, Bazzan M, Gugliotta L, et al. Diagnosis, pathogenesis and treatment of the myeloproliferative disorders essential thromboythemia, polycythemiavera and essential megakaryocytic granulocytic myeloproliferation and myelofibrosis. Neth J Med. 1999; 54: 46-62. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10079679
- Michiels JJ, Juvonen E. Proposal for revised diagnostic criteria of essential thrombocythemia and polycythemia vera by the Thrombocythemia Vera Study Group. Sem Thromb Hemost. 1997; 23: 339-347. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9263350
- Kurnick JE, Ward HP, Block MH. Bone marrow sections in the differential diagnosis of polycythemia. Arch Pathol. 1972; 94: 489-499. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/5086062
- Ellis JT, Silver RT, Coleman M, Geller SA. The bone marrow in polycythemia vera. Sem Hematol. 1975; 12: 433-444. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1198128
- Michiels JJ, Abels J, Steketee J, van Vliet HH, Vuzebvski VD. Erythromelalgia caused by platelet-mediated arteriolar inflammation and thrombosis in thrombocythemia. Ann Intern Med. 1985; 102: 466-471. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3977194
- Bellucci S, Michiels JJ. The role of JAK2V617F mutation, spontaneous eryhtropoiesis and megakaryopoiesis, hypersensitive platelets, activated leukocytes, and endothelial cells in the etiology of thrombotic manifestations in polycythemia vera and essential thrombocythemia. Sem Thromb Hemost. 2006; 32: 381-398.
- Michiels JJ. Platelet-dependent and aspirin-responsive arterial thrombophilia in essential thrombocythemia. The Thoraxcentre J. 1996; 8: 1-4.
- Michiels JJ. Bone marrow histopathology and biological markers as specific clues to the differential diagnosis of essential thrombocythemia, polycythemiavera and prefibrotic or fibrotic myeloid metaplasia. Hematol J. 2004; 5: 93-102. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15048058
- Michiels JJ, De Raeve H, Hebeda K, Lam KH, Bot F, et al. Biology Diagnosis and Classification of MPD. Furst International Lymphoma-Leukemia-Myeloma (LLM) Congress. Turk J Hematol. 2007; 24: 37-53.
- Michiels JJ, Berneman Z, Schroyens W, De Raeve H. PVSG and WHO vs European Clinical, Molecular and Pathological (ECMP) criteria for prefibrotic myeloproliferative neoplasms. World J Hematol. 2013b; 2: 71-88.
- Michiels JJ, Ten Kate FWJ, Koudstaal PJ, Van Genderen PJJ. Aspirin responsive platelet thrombophilia in essential thrombocythemia and polycythemiavera. World J Hematol. 2013c; 2: 20-43.
- Michiels JJ, Ten Kate F, Lam KH, Schroyens W, Berneman Z, et al. The European clinical, Molecular and Pathological (ECMP) criteria and the 2007/2008 revision of the World Health Organization for the diagnosis, classification and staging of prefibrotic myeloproliferative neoplasms carrying the JAK2V617F mutation. Turk J Hematol. 2014a; 31: 239-254.
- Michiels JJ, Stasko J, Kubish P, Pich A, De Raeve H. Autosomal dominant hereditary essential thrombocythemia due to a gain of function mutation in the thrombopoietin (TPO) of JAK2 gene as the cause of dominant congenital aspirin sticky platelet syndrome. J Hematol Thromb Dis. 2014b; 2: 6.
- Michiels JJ, Pich A, De Raeve H, Campr V, Schwarz J. WHO clinical molecular and pathological (WHO-CMP) features of congenital MPLS505N and the acquired MPLW515L/K mutated essential thrombocythemia and myelofibrosis. J Hematol Thromb Dis. 2014c; 2: 6.
- Michiels JJ. Myeloproliferative and thrombotic burden and treatment outcome in thrombocthemia and polycythemia patients. World J Crit Care Med. 2015; 4: 230-239. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26261774
- Michiels JJ, Berneman Z,Schroyens W, De Raeve H. Changing concepts of diagnostic criteria of myeloproliferative disorders and the molecular etiology and classification of myeloproliferative neoplasms: From Dameshek 1950 to Vainchenker 2005 and beyond. Acta Haematol. 2015a; 133: 71-86. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25116092
- Michiels JJ, Valster F, Wielenga J, Schelfout K, De Raeve H. European vs 2015 World Health Organization clinical molecular and pathological classification of myeloproliferative neoplasms. World J Hematol. 2015b; 4: 16-53.
- Michiels JJ, Medinger M De Raeve H, Schroyens W, Schelfout K, Potters V, et al. Increased erythrocyte count on top of bone marrow histology, but not by EPO level or JAK2V617F mutation load discriminates between JAK2V617F mutated essential thrombocythemia and polycythemia vera. J Hematol Thromb Dis. 2015c; 001.
- Michiels JJ, Tevet M, Trifa A, Niculescu-Mizil E, Lupa A, et al. 2016 WHO Clinical Molecular and Pathological Criteria for Classification and Staging of Myeloproliferative Neoplasms (MPN) Caused by MPN Driver Mutations in the JAK2, MPL and CALR Genes in the Context of New 2016 WHO Classification: Prognostic and Therapeutic Implications. MAEDICA. 2016; 11: 5-25. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28465746
- Michiels JJ. Aspirin-responsive eythromelalgia in JAK2-thrombocythemia and incurable inherited erythrothermalgia in neuropathic Naav1.7 sodium channellopathy: from Mitchel 1878 to Michiels 2017. Exp Opinon Orphan Drug. 2017c.
- Michiels JJ. Aspirin cures erythromelalgia and cerebrovascular distubances in JAK2-thrombocythemia. World J Hematol. 2017d; 6: 32-54.
- Wasserman LR. The management of polycythaemia vera. Br J Haematol. 1971; 21: 371-376. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/4941523
- Berlin NI. Diagnosis and classification of the polycythemias. Sem Hematol. 1975; 12: 339-351.
- Tefferi A, Pardanani A. Mutation screening for JAK2V617F: when to order the test and how to interpret the results. Leuk Res. 2006; 108: 3472-3476. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16460800
- Tefferi A, Pardanani A. Mutation screening for JAK2V617F: when to order the test and how to interpret the results. Leuk Res. 2006; 108: 3472-3476. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16460800
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127: 2391-2405. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31659364
- Michiels JJ. The Myeloproliferative Disorders. An historical appraisal and personal experiences. Leuk Lymphoma. 1996; 22: 1-14. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8951768
- Messinezy M, WestwoodNB, Woodstock SP, Strong RM, Pearson TC. Low seru, erythropoietin: a strong diagnostic criterion of primary polycythemia even et normal hemoglobin levels. Clin Lab Haematol. 1995; 17: 217-220.
- Messinezy M, Westwood NB, El-Hemaida I, Marsden JT, Sherwood RS, et al. Serum erythrpoietin values in erythrocytoses and in primary thrombocythemia. Br J Haematol. 2002; 117: 47-53. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11918532
- 2008 WHO criteria for polycthemia vera, primary myelofibrosis and essential thrombocythemia. Thiele et al In: Swerdlow SH, Campo E, Harris NL et al: WHO Classification of Tumours of Haematopoietic and Lympoid Tissues. Lyon France IARC. 2008; 40-50.
- Thomas DJ, du Boulay GH, Marshall J, Pearson TC, Ross Russell RW, et al. Cerebral blood-flow in polycythaemia. Lancet.1977; 2: 161-163. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/69781
- Thomas DJ, Marshall J, Russell RW, Wetherley-Mein G, du Boulay GH, et al. Effect of haematocrit on cerebral blood-flow in man. Lancet. 1977; 2: 941-943. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/72286
- Pearson TC, Wetherly-Mein. Vascular occlusive episodes and venous haematocrit in primary proliferative polycythaemia. Lancet. 1978; 2: 1219-1222. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/82733
- Messinezy M, Pearson TC, Prochazka A, Wetherley-Mein G. Treatment of primary proliferative polycythaemia by venesection and low dose busulphan: retrospective study from one centre. Br J Haematol. 1985; 61: 657-666. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/4084455
- Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med.2004; 350: 114-124. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14711910
- Marchioli R, Finazzi G, Vannucchi AM, Barbui T for the CTYO-PV Collaborative Group. Cardiovascular events and intensivity of treatment in polycythemia vera. N J Eng Med. 2013; 368: 22-33.
- Van Genderen PJJ, Michiels JJ. Hydroxyurea in essential thrombocytosis. N Engl J Med. 1995; 333: 802-803. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7643898
- Lengfelder E, Merx K, Hehlmann R. Diagnosis and therapy of polycythemia vera. Sem Thromb Hemost. 2006; 32: 267-275. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16673281
- Prchal JF, Axelrad AA. Bone marrow responses in polycythemia vera. N Eng J Med. 1974; 290: 1382. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/4827655
- Westwood NB, Person TC. Diagnostic applications of haematopietic progenitor culture tehniques in polycythaemias andthrombocythaemias. Leuke Lymphoma. 1996; 22: 95-103. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8951779
- Kralovics R, Guan Y, Prchal JT. Acquired uniparetal disomy of chromosome 9p is a frequent stem cell defect in polycythemia vera. Exp Hematol. 2002; 30: 229-236. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11882360
- James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, et al. A unique clonal JAK2 mutation leading to constitutive signaling causes polycythemia vera. Nature. 2005; 434: 1144-1148. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15793561
- Kralovics R, Passamonti F, Buser AS, Teo SS, Teidt R, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Eng J Med. 2005; 352: 1779-1790. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15858187
- Vainchenker W, Constantinescu SN. A unique activating mutation in JAK2 V617F is at the origin of polycythemia vera and allows a new classification of myeloproliferative diseases. Hematology Am Soc Hematol Educ Program. 2005; 195-200. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16304380
- Villeval JL, James C, Pisani DF, Casadevall N, Vainchenker W. New insights into the pathogenesis of JAK2V617F-positive myeloproliferative disorders and consequences for the management of patients. Sem Thromb Hemost. 2006; 32: 341-351.
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton et al. Acquired mutation of the tyrosine kinase in human myeloproliferative disorders. Lancet. 2005; 365: 1054-1061. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15781101
- Campbell PJ, Baxter EJ, Beer PhA, Scott LM, Bench AJ, et al. Mutation of JAK2 in the myeloproliferative disorders: timing, clonality studies, cytogenetic associations, and the role in leukemic transformation. Blood. 2006; 18: 3548-3555. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16873677
- Moliterno AR, Williams DM, Isaacs MA, Spivak JL. Phenotypic variability within the JAK2V617F-positive MPD: roles of progenitor cell and neutrophil allele burden. Exp Hematol. 2008; 36: 1480-1486. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18723264
- Vainchenker W, Delhommeau F, Constantinescu SN, Bernard OH. New mutations and pathogenesis of myeloproliferative neoplasms. Blood. 2011; 118: 1723-1735. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21653328
- Vainchenker W, Kralovics R. Genetic basis and molecular pathogenesis of classical myeloproliferative neoplasms. Blood. 2017; 129: 667-679. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28028029
- Pich A, Riera L, Beggiato E, Nicolino B, Godio L, et al. JAK2V617F mutation and allele burden are associated with distinct clinical and morphological subtypes in patients with essential thrombocythemia. J Clin Pathol. 2012; 65: 953-954. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22718845
- Antonioli E, Guglielmelli P, Pancrazzi A, Bogani C, Verrucci M, et al. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia. 2005; 19: 1847-1849. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16079890
- Gale RE, Allen AJR, Nash MJ, Linch DC. Log-term serial analysis of X-chromosome inactivation patterns andJAK2 V617F mutant levels in patients with essential thrombocythemia show that minor mutant-positive clones can remain stable for many years. Blood. 2007; 109: 1241-1243. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17023581
- Vannucchi AM, Antonioli E, Guglielmelli P, Longo G, Pancrazzi A, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2V617F allele burden. Leukemia. 2007; 21: 1952-1959. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17625606
- Passamonti F, Rumi E, Pietra D, Della Porta MG, Boveri E, et al. Relation between JAK2V617F mutation status, granulocyte activation, and constitutive mobilization of CD34+ cells into peripheral blood in myeloproliferative disorders. Blood. 2006; 107: 3676-3682. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16373657
- Scott LM, Scott MA, Campbell PJ, Green AR. Progenitors homozygous for the V617F JAK2 mutation occur in most patients with polycythemia vera, but not essential thrombocythemia. Blood. 2006; 108: 2435-2437. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16772604
- Delhommeau F, Pisani DF, James C, Casadevall N, Constatinescu S, et al. Oncogenic mechanism in myeloproliferative disorders. Cell Mol Life Sci. 2006; 63: 2939-2953.
- Mead AJ, Rugless MJ, Jacobsen SE, Schuh A. Germline JAK2 mutationin a family with hereditary thrombocytosis. N Eng J Med. 2012; 366: 967-969. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22397670
- Mead AJ, Chowdhury O, Pecquet C, Dusa A, Woll P, et al. Impact of isolatedgermline JAK2V617I mutation on human hematopoiesis. Blood. 2013; 121: 4156-4165. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23535062
- Etheridge SL, Cosgrove ME, Sangkhae V, Corbo LM, Roh ME, et al. A novel activating, germ line JAK2 mutation, JAK2R564Q, causes familialessential thrombocytosis. Blood. 2014; 123: 1059-1068. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24381227
- Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, et al. Jak2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcome. Blood. 2014; 123: 1552-1515. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24366362
- Lamy T, Devillers A, Bernard M, Moisan A, Grulois I, et al. In apparent polycythemia vera: an unrecognized diagnosis. Am J Med. 1997; 102: 14-20. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9209196
- Godfrey AL, Chen E, Pagano F, Ortmann CA, Silber Y, et al. JAK2V617F homozygosity arises commonly and recurrently in PV and ET, but PV is characterized by expansion of a dominant homozygous subclone. Blood. 2012; 120: 2704-2707. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22898600
- Bench AJ, Nacheva EP, Champion KM, Green AR. Molecular genetics and cytogenetics of myeloproliferative disorders. Baillieres Clin Haematol. 1998; 11: 819-848. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10640219
- Vannucchi A, Lasho TL, Guglielmelli P, Biamonte F, Pardani A, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013; 27: 1861-1869. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23619563
- Guglielmelli P, Lasho TL, Rotunno G, JScore J, Mannarelli C, et al. The number of prognostically detrimental mutations and prognosis in primary myelofibrosis: an international study of 797 patients. Leukemia. 2014; 28: 1494-1500. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24549259
- Lundberg P, Karov A, Nienbold R, Looser R, Hao-Shen H, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014; 123: 2220-2228. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24478400
- Tefferi A, Laslo TL, Guglielmelli P, Finke CM, Rotunno G, et al. Targeted deep sequencing in polycythemia vera and essential thrombocythemia. Blood Adv. 2016; 1: 21-30. PubMed: https://pubmed.ncbi.nlm.nih.gov/29296692
- Tefferi A, Laslo TL, Finke CM, Elala Y, Hanson CA, et al. Targeted deep sequencing in primary myelofibrosis. Blood Adv. 2016; 1: 105-111. PubMed: https://pubmed.ncbi.nlm.nih.gov/29296803
- Ortmann CA, Kent DG, Nangalia J, SilberY, Wedge DC, et al. Effect of mutation order on myeloproliferative neoplasms. N Eng J Med. 1015; 372: 601-612. PubMed: https://pubmed.ncbi.nlm.nih.gov/25946289/
- Michiels JJ, Ten Kate FWJ, Vuzevski VD, Abels J. Histopathology of erythromelalgia in thrombocythemia. Histopathology. 1984; 8: 669-678. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9263352
- Michiels JJ, Berneman Z, Schroyens W, Koudstaal PJ, Lindemans J, et al. Platelet-mediated thrombotic complications in patients with ET: reversal by aspirin, platelet reduction, and not by Coumadin. Blood Cells Mol Dis. 2006c; 36: 199-205. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16510297
- Michiels JJ, Berneman Z, Schroyens W, Koudstaal PJ, Lindemans J, et al. Platelet-mediated erythromelalgic, cerebral, ocular, and coronary microvasculae ischemic and thrombotic manifestations in patients with essential thrombocythemia and polycythemiavera: a distinct aspirin-responsive and coumadin-resitent arterial thrombophilia. Platelets. 2006d; 17: 528-544. PubMed: https://pubmed.ncbi.nlm.nih.gov/17127481/
- Van Genderen PJJ, Michiels JJ, Van Strik R, Lindemans J, Van Vliet HHDM. Platelet consumption in thrombocythemia complicated by erythromelalgia: reversal by aspirin. Thromb Haemost. 1995; 73: 210-214. PubMed: https://pubmed.ncbi.nlm.nih.gov/7792731/
- Van Genderen PJJ, Lucas IS, Van Strik R, Vuzevski VD, Prins FJ, et al. Erythromelalgia in essential thrombocythemia is characterized by platelet activation and endothelial cell damage but not by thrombin generation. Thromb Haemost. 1996; 76: 333-338. PubMed: https://pubmed.ncbi.nlm.nih.gov/8883266/
- Van Genderen PJJ, Michiels JJ. Erythromelalgia: a pathognomonic microvascular thrombotic complication in essential thrombocythemia and polycythemia vera. Sem Thromb Hemost. 1997; 23: 357-363. PubMed: https://pubmed.ncbi.nlm.nih.gov/9263352/
- Van Genderen PJJ, Mulder PGH, Waleboer M, Moesdijk D, Michiels JJ. Prevention and treatment of thrombotic comlications in essential thrombocythemia: efficacy and safety of aspirin. Brit J Haematol. 1997a; 97: 179-184. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9136963
- Van Genderen PJJ, Leenknegt H, Michiels JJ. The paradox of bleeding and thrombosis in thrombocythemia: is von Willebrand factor the link? Sem Thromb Hemost. 1997b; 23: 385-389. PubMed: https://pubmed.ncbi.nlm.nih.gov/16977569/
- Van Genderen PJJ, Prins F, Michiels JJ, Schroer K. Thromboxane-dependent platelet activation in vivo precedes arterial thrombosis in thrombocythemia: A rationale for the use of low-dose aspirin as an antithrombotic agent. Br J Haematol. 1999; 104: 438-441. PubMed: https://pubmed.ncbi.nlm.nih.gov/10086775/
- Scott LM. TheJAK2 exon 12 mutants, a comprehensive review. Amer J Hematol. 2011; 86: 668-676. PubMed: https://pubmed.ncbi.nlm.nih.gov/21674578/
- Pardani A, Lasho TL, Finke C, Hanson CA, Tefferi A. Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia vera. Leukemia. 2007; 21: 1960-1963. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17597810
- Lakey MA, Pardani A, Hoyer JD, Nguyen PL, Lasho TL, et al. Bone marrow morphologic features in polycythemia vera with JAK2 exon 12 mutations. Am J Clin Pathol. 2010; 133: 942-948. PubMed: https://pubmed.ncbi.nlm.nih.gov/20472853/
- Passamonti F, Elena C, Schnittger S, Skoda R, Anthony R Green et al. Molecular and clinical features of the myeloproliferative neoplasms associated with JAK2 exon 12 mutations. Blood. 2011; 117: 2813-2816. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21224469
- Scott LM, Tong W, Levine RL, Scott MA, Beer PA, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Eng J Med.2007; 356: 459-468. PubMed: https://pubmed.ncbi.nlm.nih.gov/17267906/
- Kim Y, Park J, Jo I, Lee GD, Kim J, et al. Genetic-pathologic characterization of myeloproiferative neoplasms. Exp Mol Med. 2016; 48: 247. PubMed: https://pubmed.ncbi.nlm.nih.gov/27444979
- Vannucchi AM, Antonioli E, Guglielmelli P, Pancrazzi A, Guerini V, et al. Charateristics and clinical correlates of MPL515W>L/K mutation in essential thrombocythemia. Blood. 2008; 112: 844-847. PubMed: https://pubmed.ncbi.nlm.nih.gov/18519816/
- Beer PA, Campbell PJ, Scott LM. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood. 2008; 112: 141-149. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18451306
- Jones AV, Campbell PJ, Beer PA, Schnittger, Vannucchi AM, et al. The JAK2 46/1 haplotype predisposes to MPL-mutated myeloproliferative neoplasms. Blood. 2010; 115: 4517-4523. PubMed: https://pubmed.ncbi.nlm.nih.gov/20304805/
- Michiels JJ, Berneman Z, Schroyens W, KuttiJ, Swolin B, et al. Philadelphia (Ph) chromosome positive thrombocythemia without features of chronic myeloid leukemia in peripheral blood and bone marrow: natural history and diagnostic differentiation from Ph-negative essential thrombocythemia. Ann Hematol. 2004; 83: 504-512. PubMed: https://pubmed.ncbi.nlm.nih.gov/15164229/
- Klampf T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, et al. Somatic mutations od calreticulin in myeloproliferative neoplasms. N Eng J Med. 2013; 369: 2379-2387. PubMed: https://pubmed.ncbi.nlm.nih.gov/24325356/
- Nangalia J, Massie CE, Baxter J, Nice FL, Gundem G, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014; 28: 1472-1477. PubMed: https://pubmed.ncbi.nlm.nih.gov/24402162/
- Rotunno G, Mannarelli C, Guglielmielli P, Pacilli A, Pancrazzi A, et al. Impact of calreticulin mutations on clinical and haematological phenotype and outcome in essential thrombocthemia. Blood. 2014; 123: 1552-1555. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24371211
- Andrikovics H, Krahling T, Balassa K, Halm G, Bors A, et al. Distinct clinical characteristics of myeloproliferative neoplasms with calreticulin mutations. Haematologica. 2014; 99: 1184-1190. PubMed: https://pubmed.ncbi.nlm.nih.gov/24895336/
- Thiele J, Kvasnicka HM, Facchetti F, Franco V, Van Der Walt J Orazi A. European consensus for grading bone marrow fibrosis and assessment of cellularity in myeloproliferative disorders. Haematologica. 2005; 90: 1128-1132. PubMed: https://pubmed.ncbi.nlm.nih.gov/16079113/
- Kiladjian JJ, Cassinat B, Turlure P, Cambier N, Roussel M, et al. High molecular response rate of polycythemia vera treated with peglyated interpheron-alpha-2a. Blood. 2006; 108: 2037-2040. PubMed: https://pubmed.ncbi.nlm.nih.gov/16709929/
- Larssen TS, Moeller MB, de Striker K, Peter Nørgaard, Jan Samuelsson, et al. Minimal residual disease and normalization of the bone marrow after longterm treatment with alfa-interferon2b in polycythemia vera. A report on molecular responses in seven patients in sustained complete hematological remission. Hematology. 2009; 14: 331-334. PubMed: https://pubmed.ncbi.nlm.nih.gov/19941739/
- Verger E, Cassinat B, Chauveau A, Dosquet C, Giradier S, et al. Clinical and moelucular response to interferon-alpha therapy in essential thrombocythemia patients with CALR mutations. Blood. 2015; 126: 2585-2691. PubMed: https://pubmed.ncbi.nlm.nih.gov/26486786/
- Wilkins BS, Erber WN, Bareford D, Buck G, Wheatley K, et al. Bone marrow pathology in essential thrombocythemia: interobserver reliability and utility for identifying disease subtypes. Blood. 2008; 111: 60-70. PubMed: https://pubmed.ncbi.nlm.nih.gov/17885079/
- James C, Delhommeau F, Marzac C, Teyssandier I, Le Couédic JP, et al. Detection of JAK2 V617F as a first intention diagnostic test for erythrocytosis. Leukemia. 2006; 20: 350-353. PubMed: https://pubmed.ncbi.nlm.nih.gov/16341032/
- Cassinat B, verger E, Kiladjian JJ. Interferon alpha therapy in CALR-mutated essential thrombocythemia. N Eng J Med. 2014; 371: 188-189. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25006741
- Juvonen E, Ikkala E, Fyrquist F, Ruutu T. Autosomal dominant erythrocytosis caused by increased sensitivity to erythropoietin. Blood. 1991; 78: 3066-3069. PubMed: https://pubmed.ncbi.nlm.nih.gov/1954391/
- De La Chapelle A, Traskelin AL, Juvonen E. Truncated erythropietin receptor causes doinantly inheritedbenign erythrocytosis. Proc Natl Acad Sci USA. 1993; 90: 4495-4499. PubMed: https://pubmed.ncbi.nlm.nih.gov/8506290/
- Lacout C, Pisani DF, Tulliez M, Gachelin FM, Vainchenker W, et al. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006; 108: 1652-1660. PubMed: https://pubmed.ncbi.nlm.nih.gov/16670266/
- Laszlo J. Myeloproliferative disorders (MPD): myelofibrosis, myelosclerosis, extramedullary hematopoiesis, undifferentiated MPD and hemorrhagic thrombocythemia. Semin Hematol. 1975; 12: 409-432. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1105793
- Quintas-Cardama A, Kantarjian H, Manshouri T, Rajyalakshmi Luthra, Zeev Estrov, et al. Peglyated interferon alfa-2a yields high rates of hematological and molecular response in patients with advanced essential thrombocthemia and polycythemia vera. J Clin Oncol. 2009; 27: 5418-5424. PubMed: https://pubmed.ncbi.nlm.nih.gov/19826111/
- Vannucchi AM, Papaemmanuil E, Campbell PJ, and Green AR. Somatic CALR Mutations in Myeloproliferative Neoplasms with Nonmutated JAK2. N Eng J Med. 2013; 369: 2391-2405. PubMed: https://pubmed.ncbi.nlm.nih.gov/24325359/
- 2001 WHO classification of the chronic myeloproliferative diseases (CMPD) polycythemia vera, chronic idiopathic myelofibrosis essential thrombocythemia and cMPD unclassifiable. In: Jaffe SS, Harris NL, Stern A, Vardiman JW eds. WHO classification of Tumours of haematopoiesis and lymphoid tissues. Lyon, France IARC; 2001; 31-42.
Figures:
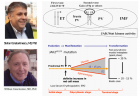
Figure 1
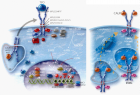
Figure 2
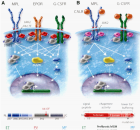
Figure 3
Figure 4
Figure 5

Figure 6
Similar Articles
-
European Clinical Laboratory, Molecular and Pathological (ECMP) criteria for prefibrotic JAK2V617F-Thrombocythemia and Polycythemia Vera versus MPL515- and CALR-Thrombocythemia and Myelofibrosis: From Dameshek to Michiels 1950-2018Jan Jacques Michiels*,Zwi Berneman,Wilfried Schroyens,Fibo W J ten Kate,King Lam,Hendrik De Raeve. European Clinical Laboratory, Molecular and Pathological (ECMP) criteria for prefibrotic JAK2V617F-Thrombocythemia and Polycythemia Vera versus MPL515- and CALR-Thrombocythemia and Myelofibrosis: From Dameshek to Michiels 1950-2018. . 2019 doi: 10.29328/journal.ijbmr.1001002; 2: 001-017
-
Primary myelofibrosis is not primary anymore since the discovery of MPL515 and CALR mutations as driver causes of mono-linear megakaryocytic and dual megakaryocytic granulocytic myeloproliferation and secondary myelofibrosisJan Jacques Michiels*,Hendrik De Raeve. Primary myelofibrosis is not primary anymore since the discovery of MPL515 and CALR mutations as driver causes of mono-linear megakaryocytic and dual megakaryocytic granulocytic myeloproliferation and secondary myelofibrosis. . 2019 doi: 10.29328/journal.ijbmr.1001003; 2: 018-026
-
The PVSG/WHO versus the Rotterdam European clinical, molecular and pathological diagnostic criteria for the classification of myeloproliferative disorders and myeloproliferative neoplasms (MPD/MPN): From Dameshek to Georgii, Vainchenker and Michiels 1950-2018Jan Jacques Michiels*,Hendrik De Raeve. The PVSG/WHO versus the Rotterdam European clinical, molecular and pathological diagnostic criteria for the classification of myeloproliferative disorders and myeloproliferative neoplasms (MPD/MPN): From Dameshek to Georgii, Vainchenker and Michiels 1950-2018. . 2019 doi: 10.29328/journal.ijbmr.1001004; 2: 027-050
-
Bone marrow histology in CALR mutated thrombocythemia and myelofibrosis: Results from two cross sectional studies in 70 newly diagnosed JAK2/MPL wild type thrombocythemia patientsJan Jacques Michiels*,Yonggoo Kim,Myungshin Kim,Francisca Valster,Vincent Potters,Zwi Berneman,Alain Gadisseur,Wilfried Schroyens,Hendrik De Raeve. Bone marrow histology in CALR mutated thrombocythemia and myelofibrosis: Results from two cross sectional studies in 70 newly diagnosed JAK2/MPL wild type thrombocythemia patients. . 2019 doi: 10.29328/journal.ijbmr.1001006; 2: 064-078
-
Novel European Asiatic Clinical, Laboratory, Molecular and Pathobiological (2015-2020 CLMP) criteria for JAK2V617F trilinear polycythemia vera (PV), JAK2exon12 PV and JAK2V617F, CALR and MPL515 thrombocythemias: From Dameshek to Constantinescu-Vainchenker, Kralovics and MichielsJan Jacques Michiels*,King H Lam,Fibo Ten Kate,Dong-Wook Kim,Myungshin Kim,Vasily Shuvaev,Francisca Valster,Vincent Potters,Wilfried Schroyens,Mihaela Andreescu,Adrian Trifa,Achille Pich,Hendrik De Raeve. Novel European Asiatic Clinical, Laboratory, Molecular and Pathobiological (2015-2020 CLMP) criteria for JAK2V617F trilinear polycythemia vera (PV), JAK2exon12 PV and JAK2V617F, CALR and MPL515 thrombocythemias: From Dameshek to Constantinescu-Vainchenker, Kralovics and Michiels. . 2020 doi: 10.29328/journal.ijbmr.1001011; 3: 001-020
Recently Viewed
-
Helping asthmatic children through bonding therapyAntonio Madrid*,Nicholas Bennett. Helping asthmatic children through bonding therapy. Arch Asthma Allergy Immunol. 2021: doi: 10.29328/journal.aaai.1001022; 5: 001-007
-
Cystic fibrosis and congenital adrenal hyperplasia: A rare occurrence with diagnostic dilemmas, similarities and contradictionsAfshin Rezaeifar*,Mohammad Nabavi,Parisa Rezaeifar,Morteza Fallahpour,Saba Arshi,Mohammad Hassan Bemanian,Sima Shokri. Cystic fibrosis and congenital adrenal hyperplasia: A rare occurrence with diagnostic dilemmas, similarities and contradictions. Arch Asthma Allergy Immunol. 2020: doi: 10.29328/journal.aaai.1001021; 4: 018-020
-
Short-term responses to high-dose inhaled corticosteroid treatment in patients with chronic obstructive pulmonary disease with a fractional nitric oxide concentration over 35 parts per billion: A single-centre pre–post studyAkihiro Shiroshita*,Yu Tanaka,Kei Nakashima,Atsushi Shiraishi,Hiroki Matsui,Masahiro Aoshima. Short-term responses to high-dose inhaled corticosteroid treatment in patients with chronic obstructive pulmonary disease with a fractional nitric oxide concentration over 35 parts per billion: A single-centre pre–post study. Arch Asthma Allergy Immunol. 2020: doi: 10.29328/journal.aaai.1001020; 4: 012-017
-
A mild form of Familial Mediterranean Fever associated with a polymorphisms C.NT 1588,-69G>Enrico Cillari*,Francesco Arcoleo,Carmelo Fabiano,Stefania Leto Barone. A mild form of Familial Mediterranean Fever associated with a polymorphisms C.NT 1588,-69G>. Arch Asthma Allergy Immunol. 2020: doi: 10.29328/journal.aaai.1001019; 4: 009-011
-
Immune system and quality of life following aerobic exercise versus resistance exercise training among Alzheimer’sFadwah M Al-Sharif*. Immune system and quality of life following aerobic exercise versus resistance exercise training among Alzheimer’s. Arch Asthma Allergy Immunol. 2020: doi: 10.29328/journal.aaai.1001018; 4: 003-008
Most Viewed
-
Impact of Latex Sensitization on Asthma and Rhinitis Progression: A Study at Abidjan-Cocody University Hospital - Côte d’Ivoire (Progression of Asthma and Rhinitis related to Latex Sensitization)Dasse Sery Romuald*, KL Siransy, N Koffi, RO Yeboah, EK Nguessan, HA Adou, VP Goran-Kouacou, AU Assi, JY Seri, S Moussa, D Oura, CL Memel, H Koya, E Atoukoula. Impact of Latex Sensitization on Asthma and Rhinitis Progression: A Study at Abidjan-Cocody University Hospital - Côte d’Ivoire (Progression of Asthma and Rhinitis related to Latex Sensitization). Arch Asthma Allergy Immunol. 2024 doi: 10.29328/journal.aaai.1001035; 8: 007-012
-
Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian RandomizationYong-Qing Zhu, Xiao-Yan Meng, Jing-Hua Yang*. Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian Randomization. Arch Asthma Allergy Immunol. 2023 doi: 10.29328/journal.aaai.1001032; 7: 012-022
-
An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergiesNathalie Cottel,Aïcha Dieme,Véronique Orcel,Yannick Chantran,Mélisande Bourgoin-Heck,Jocelyne Just. An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergies. Arch Asthma Allergy Immunol. 2021 doi: 10.29328/journal.aaai.1001027; 5: 030-037
-
Snow white: an allergic girl?Oreste Vittore Brenna*. Snow white: an allergic girl?. Arch Asthma Allergy Immunol. 2022 doi: 10.29328/journal.aaai.1001029; 6: 001-002
-
Cytokine intoxication as a model of cell apoptosis and predict of schizophrenia - like affective disordersElena Viktorovna Drozdova*. Cytokine intoxication as a model of cell apoptosis and predict of schizophrenia - like affective disorders. Arch Asthma Allergy Immunol. 2021 doi: 10.29328/journal.aaai.1001028; 5: 038-040

If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."