Abstract
Retrospective Study
Impact of Intravenous Busulfan Pharmacokinetics on Safety in Pediatric Patients who have undergone Hematopoietic Stem Cell Transplant
Omar AL Mofleh*, Noha Awadalla, Amal AL Shafi, Lina Husain, Hanan AL Musabeh and Saad AL Daama
Published: 03 December, 2024 | Volume 7 - Issue 1 | Pages: 007-012
Introduction: Busulfan (Bu)-based regimens are crucial for myeloablative conditioning in pediatric allogeneic stem cell transplantation. Despite its efficacy, Intravenous Bu has a narrow therapeutic index and variable pharmacodynamics especially in children, heightening the risk of adverse events. This study explores Bu dosing and related organ toxicities in pediatric patients at a tertiary center in Saudi Arabia.
Methodology: This retrospective study at King Fahad Specialist Hospital in Dammam (KFSH-D), Saudi Arabia, included pediatric patients (≤16 years) treated with intravenous Bu before bone marrow transplantation from 2010 to 2022. Pharmacokinetic dose adjustments were based on AUC targets of 900-1350 µMol-min. Descriptive measures included mean, Standard Deviation (SD), median, minimum-maximum values, counts, and percentages. Statistical analyses used Kruskal-Wallis, Chi-square, and Fisher’s exact tests. Ethical approval was obtained from KFSH-D.
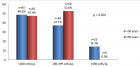
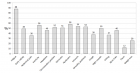
Results: We identified 44 pediatric patients who underwent Bu prior to HSCT. Mean age was 4.95 ± 2.49 years, with a female majority (56.8%). Primary diseases included Beta Thalassemia (34.09%), Neuroblastoma (29.55%) among others. There was no significant difference in the cohort’s demographic and clinical features of the cohort. Nonetheless, higher infections were found in the Low-AUC group (66.7%) compared to the Target-AUC (40.0%) and Higher-AUC groups (0.0%) (p = 0.015).
Conclusion: This study emphasizes the need for therapeutic drug monitoring and individualized Bu dosing in pediatric HSCT to minimize toxicity and improve outcomes. Larger multicenter studies are recommended to refine dosing strategies and enhance the safety and efficacy of Bu-based regimens.
Read Full Article HTML DOI: 10.29328/journal.ijbmr.1001018 Cite this Article Read Full Article PDF
Keywords:
Busulfan; Pediatrics; Adverse events; AUC; Toxicity; HSCT; Drug monitoring
References
- Kikuchi T, Mori T, Ohwada C, Onoda M, Shimizu H, Yokoyama H, et al. Pharmacokinetics of intravenous busulfan as condition for hematopoietic stem cell transplantation: comparison between combinations with cyclophosphamide and fludarabine. Int J Hematol. 2021;113(1):128-133. Available from: https://doi.org/10.1007/s12185-020-02990-y
- Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124(3):344-353. Available from: https://doi.org/10.1182/blood-2014-02-514778
- Masson E, Zamboni WC. Pharmacokinetic Optimisation of Cancer Chemotherapy. Clin Pharmacokinet. 1997;32(4):324-343. Available from: https://doi.org/10.2165/00003088-199732040-00005
- Copelan EA, Bechtel TP, Avalos BR, Elder PJ, Ezzone SA, Scholl MD, et al. Busulfan levels are influenced by prior treatment and are associated with hepatic veno-occlusive disease and early mortality but not with delayed complications following marrow transplantation. Bone Marrow Transplant. 2001;27(11):1121-4. Available from: https://doi.org/10.1038/sj.bmt.1703047
- Bleyzac N, Souillet G, Magron P, Janoly A, Martin P, Bertrand Y, et al. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant. 2001;28(8):743-51. Available from: https://doi.org/10.1038/sj.bmt.1703207
- Vassal G. Busulfan and Veno-occlusive Disease of the Liver. Ann Intern Med. 1990;112(11):881. Available from: https://doi.org/10.7326/0003-4819-112-11-881_1
- Tsakiris DA, Tichelli A. Thrombotic complications after haematopoietic stem cell transplantation: early and late effects. Best Pract Res Clin Haematol. 2009;22(1):137-145. Available from: https://doi.org/10.1016/j.beha.2008.12.002
- Hassan Z, Nilsson C, Hassan M. Liposomal busulphan: bioavailability and effect on bone marrow in mice. Bone Marrow Transplant. 1998;22(9):913-918. Available from: https://doi.org/10.1038/sj.bmt.1701458
- Andersson BS, Kashyap A, Gian V, Wingard JR, Fernandez H, Cagnoni PJ, et al. Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: a phase II study. Biol Blood Marrow Transplant. 2002;8(3):145-54. Available from: https://doi.org/10.1053/bbmt.2002.v8.pm11939604
- Kashyap A, Wingard J, Cagnoni P, Roy J, Tarantolo S, Hu W, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002;8(9):493-500. Available from: https://doi.org/10.1053/bbmt.2002.v8.pm12374454
- Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental Pharmacology — Drug Disposition, Action, and Therapy in Infants and Children. N Engl J Med. 2003;349(12):1157-1167. Available from: https://doi.org/10.1056/nejmra035092
- Myers AL, Kawedia JD, Champlin RE, Kramer MA, Nieto Y, Ghose R, et al. Clarifying busulfan metabolism and drug interactions to support new therapeutic drug monitoring strategies: a comprehensive review. Expert Opin Drug Metab Toxicol. 2017;13(9):901-923. Available from: https://doi.org/10.1080/17425255.2017.1360277
- Nava T, Kassir N, Rezgui MA, Uppugunduri CRS, Huezo-Diaz Curtis P, Duval M, et al. Incorporation of GSTA1 genetic variations into a population pharmacokinetic model for IV busulfan in paediatric hematopoietic stem cell transplantation. Br J Clin Pharmacol. 2018;84(7):1494-1504. Available from: https://doi.org/10.1111/bcp.13566
- Kim MG, Kwak A, Choi B, Ji E, Oh JM, Kim K. Effect of glutathione S‐transferase genetic polymorphisms on busulfan pharmacokinetics and veno‐occlusive disease in hematopoietic stem cell transplantation: A meta‐ Basic Clin Pharmacol Toxicol. 2019;124(6):691-703. Available from: https://doi.org/10.1111/bcpt.13185
- Ansari M, Huezo-Diaz P, Rezgui MA, Marktel S, Duval M, Bittencourt H, et al. Influence of glutathione S-transferase gene polymorphisms on busulfan pharmacokinetics and outcome of hematopoietic stem-cell transplantation in thalassemia pediatric patients. Bone Marrow Transplant. 2016;51(3):377-383. Available from: https://doi.org/10.1038/bmt.2015.321
- Hassan Z, Hellström-Lindberg E, Alsadi S, Edgren M, Hägglund H, Hassan M. The effect of modulation of glutathione cellular content on busulphan-induced cytotoxicity on hematopoietic cells in vitro and in vivo. Bone Marrow Transplant. 2002;30(3):141-147. Available from: https://doi.org/10.1038/sj.bmt.1703615
- DeLeve LD, Wang X. Role of Oxidative Stress and Glutathione in Busulfan Toxicity in Cultured Murine Hepatocytes. Pharmacology. 2000;60(3):143-154. Available from: https://doi.org/10.1159/000028359
- Feng X, Wu Y, Zhang J, Li J, Zhu G, Fan D, et al. Busulfan systemic exposure and its relationship with efficacy and safety in hematopoietic stem cell transplantation in children: a meta-analysis. BMC Pediatr. 2020;20(1):176. Available from: https://doi.org/10.1186/s12887-020-02028-6
- Warsy AS, Al-Jaser MH, Albdass A, Al-Daihan S, Alanazi M. Is consanguinity prevalence decreasing in Saudis?: A study in two generations. Afr Health Sci. 2014;14(2):314-321. Available from: https://doi.org/10.4314/ahs.v14i2.5
- Albanghali MA. Prevalence of Consanguineous Marriage among Saudi Citizens of Albaha, a Cross-Sectional Study. Int J Environ Res Public Health. 2023;20(4):3767. Available from: https://doi.org/10.3390/ijerph20043767
- Alhajori FS, Makkawi MH, Alasmari SZ, Shaikh AA, Baig MA. Estimating the prevalence of pediatric hematological malignancies in Al-Madinah Al-Munawwarah, Saudi Arabia. Saudi Med J. 2023;44(5):504-508. Available from: https://doi.org/10.15537/smj.2023.44.5.20220915
- Chaudhri E, Fathi W, Hussain F, Hashmi SK. The Increasing Trends in Cases of the Most Common Cancers in Saudi Arabia. J Epidemiol Glob Health. 2020;10(4):258-262. Available from: https://doi.org/10.2991/jegh.k.200515.001
- el-Hazmi MA, al-Swailem AR, Warsy AS, al-Swailem AM, Sulaimani R, al-Meshari AA. Consanguinity among the Saudi Arabian population. J Med Genet. 1995;32(8):623-626. Available from: https://doi.org/10.1136/jmg.32.8.623
- Hill BT. It’s Personal: Achieving Optimal Busulfan Exposure for All Patients. Biol Blood Marrow Transplant. 2016;22(7):1149-1150. Available from: https://doi.org/10.1016/j.bbmt.2016.04.024
- Neely M, Philippe M, Rushing T, Fu X, van Guilder M, Bayard D, et al. Accurately Achieving Target Busulfan Exposure in Children and Adolescents With Very Limited Sampling and the BestDose Software. Ther Drug Monit. 2016;38(3):332-342. Available from: https://doi.org/10.1097/ftd.0000000000000276
- Bartelink IH, Bredius RGM, Belitser SV, Suttorp MM, Bierings M, Knibbe CA, et al. Association between Busulfan Exposure and Outcome in Children Receiving Intravenous Busulfan before Hematologic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2009;15(2):231-241. Available from: https://doi.org/10.1016/j.bbmt.2008.11.022
- Zhou S, Zhai Y, Yan L, Shi X, Shang J, Wu D, et al. Busulfan/Cyclophosphamide Compared with Melphalan as a Conditioning Regimen for Autologous Transplantation of Multiple Myeloma: A Long-Term Assessment. J Clin Med. 2023;12(19):6239. Available from: https://doi.org/10.3390/jcm12196239
- Soiffer RJ, LeRademacher J, Ho V, Kan F, Artz A, Champlin RE, et al. Impact of immune modulation with anti–T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963-6970. Available from: https://doi.org/10.1182/blood-2011-01-332007
- Socié G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti–T-cell globulin ATG-Fresenius. Blood. 2011;117(23):6375-6382. Available from: https://doi.org/10.1182/blood-2011-01-329821
- Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, et al. Chemotherapy-Refractory Diffuse Large B-Cell Lymphoma and Indolent B-Cell Malignancies Can Be Effectively Treated With Autologous T Cells Expressing an Anti-CD19 Chimeric Antigen Receptor. J Clin Oncol. 2015;33(6):540-549. Available from: https://doi.org/10.1200/jco.2014.56.2025
- Finke J, Schmoor C, Bethge WA, Ottinger H, Stelljes M, Volin L, et al. Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-T-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: final results of a randomised controlled trial. Lancet Haematol. 2017;4(6):e293-e301. Available from: https://doi.org/10.1016/s2352-3026(17)30081-9
- Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, et al. Thymoglobulin Prevents Chronic Graft-versus-Host Disease, Chronic Lung Dysfunction, and Late Transplant-Related Mortality: Long-Term Follow-Up of a Randomized Trial in Patients Undergoing Unrelated Donor Transplantation. Biol Blood Marrow Transplant. 2006;12(5):560-565. Available from: https://doi.org/10.1016/j.bbmt.2005.12.034
Figures:
Similar Articles
-
Serum MicroRNA-155 in Acute Graft-Versus-Host-Disease (aGVHD)Yvonne A Efebera*,Amy S Ruppert,Apollinaire Ngankeu,Sabrina Garman,Prasanthi Kumchala,Alan Howard,Steven M Devine,Parvathi Ranganathan,Ramiro Garzon. Serum MicroRNA-155 in Acute Graft-Versus-Host-Disease (aGVHD). . 2019 doi: 10.29328/journal.ijbmr.1001007; 2: 079-082
-
The motivational factors and adverse events experienced by healthy volunteers donating bone marrow for researchMirella Ejiugwo,Georgina Shaw,Frank Barry,Janusz Krawczyk,Veronica McInerney*. The motivational factors and adverse events experienced by healthy volunteers donating bone marrow for research. . 2019 doi: 10.29328/journal.ijbmr.1001010; 2: 089-096
-
Impact of Intravenous Busulfan Pharmacokinetics on Safety in Pediatric Patients who have undergone Hematopoietic Stem Cell TransplantOmar AL Mofleh*,Noha Awadalla,Amal AL Shafi,Lina Husain,Hanan AL Musabeh,Saad AL Daama. Impact of Intravenous Busulfan Pharmacokinetics on Safety in Pediatric Patients who have undergone Hematopoietic Stem Cell Transplant. . 2024 doi: 10.29328/journal.ijbmr.1001018; 7: 007-012
-
Exploring the Potential of Medicinal Plants in Bone Marrow Regeneration and Hematopoietic Stem Cell TherapyUgwu Okechukwu Paul-Chima*,Alum Esther Ugo. Exploring the Potential of Medicinal Plants in Bone Marrow Regeneration and Hematopoietic Stem Cell Therapy. . 2025 doi: 10.29328/journal.ijbmr.1001019; 8: 001-005
Recently Viewed
-
In at the deep end: Psychosocial aspects of developing autonomy in histopathology trainingAI Finall*. In at the deep end: Psychosocial aspects of developing autonomy in histopathology training. Arch Pathol Clin Res. 2018: doi: 10.29328/journal.apcr.1001007; 2: 013-019
-
Pathological Effects of Cypermethrin on the Testes and Accessory Sexual Glands of Yankasa RamsUbah Simon*,Ogwu David,Rekwot Peter, Rwuaan Joseph,Chibuogwu Ijeoma, Njoku Celestine. Pathological Effects of Cypermethrin on the Testes and Accessory Sexual Glands of Yankasa Rams. Arch Pathol Clin Res. 2018: doi: 10.29328/journal.apcr.1001006; 2: 006-012
-
Predicament of classification: Multisystem small vessel vasculitis with cresentic GlomerulonephritisAwad Magbri*,Shaukat Rashid,Balhinder Brar . Predicament of classification: Multisystem small vessel vasculitis with cresentic Glomerulonephritis. Arch Pathol Clin Res. 2018: doi: 10.29328/journal.apcr.1001005; 2: 001-005
-
A great mimicker of Bone Secondaries: Brown Tumors, presenting with a Degenerative Lumber Disc like painZuhal Bayramoglu*,Ravza Yılmaz,Aysel Bayram. A great mimicker of Bone Secondaries: Brown Tumors, presenting with a Degenerative Lumber Disc like pain. Arch Pathol Clin Res. 2017: doi: 10.29328/journal.hjpcr.1001004; 1: 018-023
-
MicroRNA Therapeutics in Triple Negative Breast CancerSarmistha Mitra*. MicroRNA Therapeutics in Triple Negative Breast Cancer . Arch Pathol Clin Res. 2017: doi: 10.29328/journal.hjpcr.1001003; 1: 009-017
Most Viewed
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Evaluation of In vitro and Ex vivo Models for Studying the Effectiveness of Vaginal Drug Systems in Controlling Microbe Infections: A Systematic ReviewMohammad Hossein Karami*, Majid Abdouss*, Mandana Karami. Evaluation of In vitro and Ex vivo Models for Studying the Effectiveness of Vaginal Drug Systems in Controlling Microbe Infections: A Systematic Review. Clin J Obstet Gynecol. 2023 doi: 10.29328/journal.cjog.1001151; 6: 201-215
-
Prospective Coronavirus Liver Effects: Available KnowledgeAvishek Mandal*. Prospective Coronavirus Liver Effects: Available Knowledge. Ann Clin Gastroenterol Hepatol. 2023 doi: 10.29328/journal.acgh.1001039; 7: 001-010
-
Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian RandomizationYong-Qing Zhu, Xiao-Yan Meng, Jing-Hua Yang*. Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian Randomization. Arch Asthma Allergy Immunol. 2023 doi: 10.29328/journal.aaai.1001032; 7: 012-022
-
An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergiesNathalie Cottel,Aïcha Dieme,Véronique Orcel,Yannick Chantran,Mélisande Bourgoin-Heck,Jocelyne Just. An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergies. Arch Asthma Allergy Immunol. 2021 doi: 10.29328/journal.aaai.1001027; 5: 030-037

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."